Optimizing HPV Vaccination Strategies: Insights from Vaccine Technologies, Immune Response and Epidemiological Trends from Togo
- Post by: wsmib-admin
- septembre 30, 2024
- Comments off
Optimizing HPV Vaccination Strategies: Insights from Vaccine Technologies, Immune Response and Epidemiological Trends from Togo
Hélène1, 2, 3, Katawa Gnatoulma1 and Kolou Malewe4
1Unité de Recherche en Immunologie et Immunomodulation (UR2IM) /Laboratoire de Microbiologie et de Contrôle de Qualité des Denrées Alimentaires (LAMICODA), Ecole Supérieure des Techniques Biologiques et Alimentaires, Université de Lomé, Lomé, Togo
2West African Centre for Cell Biology of Infectious Pathogens (WACCBIP), University of Ghana, Accra, Ghana.
3Department of Biochemistry, Cell and Molecular Biology, College of Basic and Applied Sciences, University of Ghana, Accra, Ghana.
4Faculté des Sciences de la Santé, Université de Lomé, Lomé, Togo
Abstract
Human papillomavirus (HPV) is a common sexually transmitted infection, with particular high-risk strains considerably contributing to the development of cervical cancer, a leading cause of morbidity and death in women in low- and middle-income countries (LMICs). In Togo, cervical cancer is a major public health concern. In response, the government has initiated an HPV immunization program. However, detailed studies investigating the distribution of HPV types in the Togolese population are rare, which is critical for improving the vaccine program’s efficiency. This mini-review provides a comprehensive analysis of existing data on HPV prevalence and type distribution in Togo, as well as explores the implications for the newly adopted HPV vaccination program and the immune responses triggered by the various HPV vaccines. By examining current data on HPV prevalence and type distribution, this study highlights the importance of local epidemiological data in guiding vaccination choice and policy decisions.
Keywords
HPV Vaccination, Immune Response, Epidemiology, Togo
Introduction
Human papillomavirus (HPV) is one of the most common sexually transmitted infections globally, with high-risk genotypes playing a substantial role in cervical cancer development. Cervical cancer remains a prominent source of morbidity and mortality among women in low- and middle-income countries (LMICs), including several regions in Sub-Saharan Africa (1, 2).
HPV is a family of more than 200 related viruses, with over 40 genotypes known to infect the genital epithelium. These viruses are categorized into high-risk types, medium and low risk types. The high-risk types are oncogenic and associated with cervical and other anogenital malignancies, while medium and low risk cause benign lesions such us genital warts. Among the high-risk HPV types, HPV-16 and HPV-18 account approximately for 70% of cervical cancer occurrences globally. However, HPV genotypes distributed vary significantly across the different regions of the world (3, 4).
The introduction of prophylactic vaccines targeting these high-risk types has shown significant prospects in reducing the incidence of HPV infections and cervical cancer (5). Currently, three prophylactic HPV vaccines, approved by the U.S. Food and Drug Administration (FDA), are available, and countries with established HPV vaccination programs have reported significant reductions in the prevalence of vaccine-targeted HPV types and a decline in cervical cancer incidence (6, 7).
The burden of HPV and related cervical cancer in Togo (a West African country), is substantial (8). To address this public health challenge, Togo has recently initiated an HPV vaccination program to combat HPV-related diseases and reduce cervical cancer incidence (9). Understanding the HPV genotype distribution is critical for adjusting vaccination approaches and enhancing screening programs to meet the unique needs of various populations. Furthermore, factors such as geographical disparities, age, sexual behavior, and co-infections also have a substantial impact on HPV prevalence and should be addressed when evaluating vaccination efficacy. However, intensive investigations focusing on HPV-type distribution in Togo are scarce.
The implementation of an HPV vaccination program in Togo is a significant step towards reducing the burden of HPV-related conditions. However, the success of this program relies on a comprehensive overview of the local HPV epidemiology. Detailed data on HPV type distribution in Togo is critical to ensure that the vaccination program targets the most prevalent and high-risk HPV types within the given population, thereby increasing its efficacy.
This review aims to provide a comprehensive analysis of the distribution of HPV types in Togo and discuss the implications for the ongoing HPV vaccination program, as well as the immune response triggered by the available HPV vaccines. Understanding the prevalence and distribution of various HPV types in Togo might assist policymakers, public health officials, and stakeholders in identifying the most effective vaccination strategies for reducing the burden of HPV-related diseases and, ultimately, improving women’s health outcomes in Togo.
Methods
A comprehensive search of databases, including PubMed, Google Scholar, ELSEVIER, WHO, International Human Papillomavirus Reference Center (IHPRC) and African Journals Online (AJOL), was performed using the following key terms: “HPV prevalence in Togo,” “HPV type distribution in Togo,” “cervical cancer in Togo,” “HPV vaccination in Africa,” “HPV vaccines technologies,” “HPV vaccines AND immune response,” and “HPV immunization programs in low- and middle-income countries (LMICs).” Articles, reports, and official health papers published until 2024 were curated. The inclusion criteria were papers that reported HPV prevalence, genotyping data, HPV vaccines technology, immunes response in HPV vaccines, and cervical cancer epidemiology in Togo or other relevant West African nations. The assessment omitted studies that did not provide clear HPV type-specific data or were not directly relevant to the Togolese population. Furthermore, duplicate papers and research not related to cervical cancer or HPV vaccination programs were excluded. The selected studies included key information such as sample size, HPV prevalence rates, HPV type distribution, and cervical cancer incidence in Togo. The retrieved data were integrated and analyzed to highlight the HPV burden in Togo, HPV vaccine’s immune response with an emphasis on the prevalence of high-risk HPV strains compared to those addressed by the country’s existing vaccinations. The findings’ implications were then examined in terms of public health initiatives, such as vaccine policy optimization. Finally, the evaluation highlighted gaps in the existing data and prioritized future research to increase HPV vaccine coverage and efficacy in Togo. The literature review process is summarised in figure 1.
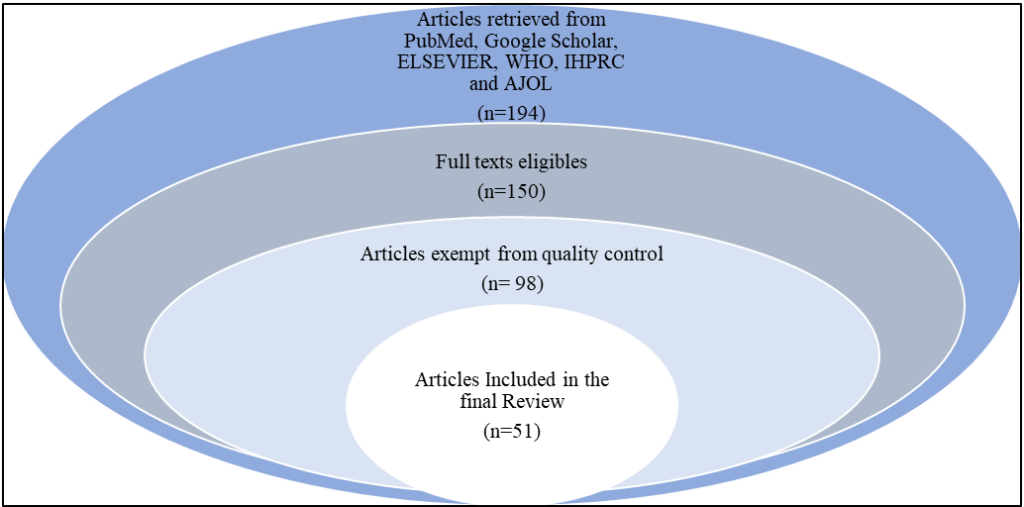
Figure 1: Flowchart of data curation process; A total of 194 publications were retrieved from various databases, including PubMed, Google Scholar, ELSEVIER, WHO, IHPRC, and AJOL. 150 articles have been selected based on the availability of full texts. Following a thorough evaluation of the entire texts, 98 articles fulfilled the inclusion criteria. Ultimately, 51 articles met all the eligibility requirements and were included in the final review.
HPV Vaccines technologies
Human papillomavirus (HPV) vaccines are a crucial tool in the fight against HPV-related diseases, including cervical cancer, genital warts, and other anogenital cancers. Since the first HPV vaccine was introduced in 2006, advancements in vaccine technology have expanded the scope and efficacy of HPV vaccination programs worldwide (10). Three highly effective prophylactic HPV vaccines licensed and approved by U.S. Food and Drug Administration (FDA) are designed to protect against two, four, or nine types of persistent HPV infections (11). These vaccines have demonstrated significant efficacy in reducing the burden of cervical cancer (CC) in developed countries (12). The bivalent vaccine, Cervarix™ (developed by GlaxoSmithKline), offers protection against HPV types 16 and 18, which are strongly associated with cervical and other anogenital cancers. The quadrivalent vaccine, Gardasil 4®, targets HPV types 6, 11, 16, and 18. HPV Types 6 and 11 are primarily responsible for the majority of genital warts cases. Furthermore, the nonavalent vaccine, Gardasil 9® extends its protection to five additional HPV types (31, 33, 45, 52, and 58), further broadening the spectrum of prevention against cervical and other related cancers (13). The Gardasil vaccines are developed by Merck (Table 1). The widespread use of these vaccines has resulted in a substantial decrease in the incidence of HPV-related diseases, then significantly transformed the landscape of public health concerning HPV-related diseases. This transformation is particularly evident in the reduction of HPV infections, cervical cancer rates, and other HPV-associated conditions underscoring their critical role in public health (14, 15).
Table 1: Available HPV vaccines and their immunogenicity
| Features | CervarixTM | Gardasil 4® | Gardasil 9® |
| Manufacturer | GlaxoSmithKline, GSK, Brentford, | Kenilworth, NJ, USA Merk and Co, | Kenilworth, NJ, USA Merk and Co, |
| UK., | Inc., | Inc., | |
| Antigen | L1 VLP | L1 VLP | L1 VLP |
| HPV genotypes | HPV 16, 18 | HPV 6, 11, 16, 18 | HPV 6, 11, 16, 18, 31, 33, 45, 52, 58 |
| Adjuvant | Aluminum salt + TSL agonist (monophosphoryl lipid A) | Aluminium salt | Aluminium salt |
| Additional ingredients | Sodium chloride | Sodium chloride Polysorbate 80 | Sodium chloride |
| Polysorbate 80 | L-histidine | Polysorbate 80 | |
| Sodium dihydrogen phosphate dehydrate | Sodium borate | L-histidine | |
| Water for injection | Water for injection | Sodium borate | |
| Water for injection | |||
| Immune response | Specific CD4 T cells | Specific CD4 T cells | Broadly neutralizing antibody |
| Broadly neutralizing antibody | Broadly neutralizing antibody | Specific CD4 T cells | |
| IL-2 and TNF-α | IL-2, IFN- γ and TNF-α | IL-2 and IFN- γ | |
| Ref | (16-18) | (19-22) | (11, 18, 19, 23, 24) |
All HPV vaccines comprise virus-like particles (VLPs) which are based on the major HPV capsid protein L1 from the specific HPV types. HPV L1 protein can self-assemble into virus-like particles (VLPs) that mimic the structure of the native virus but do not contain viral DNA, making them non-infectious (25). HPV vaccines are made from different technological approaches (Figure 2). The recombinant proteins forming the virus-like particle (VLPs) are produced by separate fermentation in different expression systems, a baculovirus expression system was used for the bivalent vaccine while the recombinant Saccharomyces cerevisiae was used for the Gardasil vaccines (5). The quadrivalent and nonavalent vaccines utilize only aluminum salts as an adjuvant, while, VLPs of Cervarix are adsorbed on aluminum hydroxy phosphate sulfate adjuvant (AS04), which includes aluminum hydroxide salts and the Tall-like receptor (TLR-4) agonist MPL (3-O-desacyl-4’-monophosphoryl lipid A). Additionally, Cervarix contains sodium dihydrogen phosphate dehydrate, polysorbate 80, sodium chloride, and water for injection. In contrast, both Gardasil vaccine’s formulations include sodium chloride, L-histidine, polysorbate 80, sodium borate, and water. The final product is presented as a sterile suspension in a single-dose vial for intramuscular injection (26). WHO recommends the vaccination should start at the age of 9, early at the onset of sexual activities, however, the recommended dosage and schedule for HPV vaccines depends on the age at which the vaccination series is initiated. For girls aged between 9 and 14, a 2-dose series is recommended, with the second dose given 6 to 12 months after the first dose. The minimum interval between the two doses is 5 months. For 15-year-old and beyond ladies; a 3-dose series is recommended, with the second dose given 1 to 2 months after the first dose, and the third dose given 6 months after the first dose. The minimum intervals are 4 weeks between the first and second dose. Lastly, a 3-dose series is recommended for those with immunocompromising conditions, including HIV infection (27).
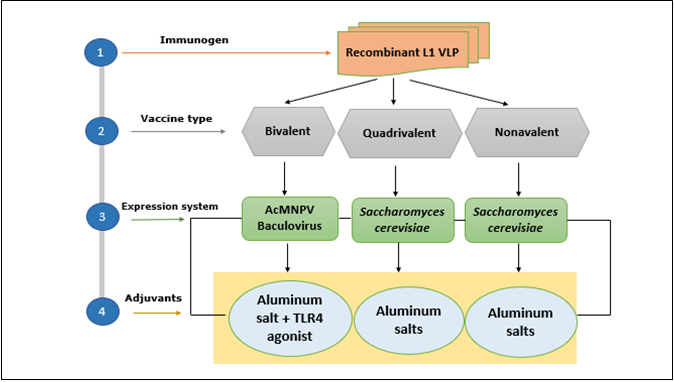
Figure 2: HPV Vaccine strategies; HPV vaccines rely on vaccines made from virus-like particles (VLPs), which are produced through fermentation in various expression systems. The bivalent vaccine (Cervarix) uses a baculovirus expression system, while the Gardasil vaccines are produced using yeast (Saccharomyces cerevisiae). The VLPs in the bivalent vaccine are adsorbed onto an adjuvant system composed of amorphous aluminum hydroxyphosphate sulfate, whereas the quadrivalent and nonavalent vaccines utilize only aluminum salts as an adjuvant.
Immune response against HPV Vaccines
HPV vaccines are formulated to prevent infections by high-risk human papillomavirus (HPV) types, notably HPV 16 and 18, which are causally linked to cervical cancer and other malignancies. These vaccines utilize virus-like particles (VLPs) that structurally mimic the viral capsid while being non-infectious, thereby eliciting a robust immune response (28). Vaccination induces high titers of neutralizing antibodies, particularly IgG, which effectively block viral entry into host cells. This immune response is durable, with studies demonstrating sustained antibody levels over extended periods, negating the need for booster doses (29, 30). Additionally, certain vaccines confer partial cross-protection against non-vaccine HPV types through structural similarity. While systemic antibody responses are central to protection, mucosal immunity is critical, as vaccine-induced IgG transudates into cervical mucus, offering frontline defense at the primary sites of HPV infection (31). When administered before HPV exposure, typically in adolescence, these vaccines have shown exceptional efficacy in significantly reducing the incidence of HPV-related diseases (32).
CervarixTM generates a strong and durable immune response, marked by high levels of neutralizing antibodies and HPV-specific CD4+ T cells, which are likely key to its high efficacy in preventing HPV infection and related diseases. The vaccine induces a robust immune response against HPV types 16 and 18, with over 99% of initially seronegative individuals seroconverting to both HPV-16 and HPV-18 one month after the third dose (33). Specific IgG antibody levels peak around month 7 and subsequently decline to a plateau that remains at least 10 times higher than levels induced by natural infection, persisting for up to 113 months (9.4 years) after the first dose (34). Cervarix has also been shown to produce significantly higher serum levels of neutralizing antibodies, specifically IgG, against HPV-16 and HPV-18 compared to Gardasil after administering three doses (17, 18). Moreover, Cervarix induces higher frequencies of CD4+ T cells producing IL-2 in response to HPV-18 L1, along with a general trend toward elevated frequencies of CD4+ T cells producing IL-2 and TNF-α for HPV16, 18, 33, and 45. In addition, Cervarix appears to offer greater cross-protection against non-vaccine HPV types, before the development of Gardasil 9® particularly HPV-31 and HPV-45, compared to Gardasil 4® (18).
The Gardasil 4® vaccine elicits a robust and sustained immune response, against four HPV types (6, 11, 16, and 18) through the production of high levels of neutralizing antibodies, which are key to preventing infection. Upon vaccination, nearly all individuals seroconvert, achieving seropositivity rates of 96.4% to 99.9%, with peak antibody titers typically observed around Month 7. These titters, represented as geometric mean titers (GMTs), remain significantly elevated compared to natural infection levels, although a decline over time is expected. Notably, age-related differences in GMTs are observed, with younger individuals generally exhibiting higher titers, reflecting the stronger immune responses typically seen in younger populations (19). In addition to humoral responses, Gardasil 9® also promotes a significant T cell-mediated immune response, contributing to its protective efficacy against HPV-related diseases. This includes the activation of CD4+ T cells, which play a critical role in sustaining antibody production and enhancing immune memory (20). Gardasil 4 elicits the production of cytokines like IL-2, INF-γ, and TNF-α, indicating a strong Th1-type immune response, which is important for long-term protection (21). Studies have shown that the antibodies produced are highly effective at neutralizing live HPV virions, further underscoring the vaccine’s protective capacity (22). Over time, antibody levels naturally decline; however, long-term studies indicate that the immune memory remains intact. When individuals receive a booster dose several years later, there is a rapid and robust increase in antibody titters, demonstrating the presence of an anamnestic response (35). This memory response is crucial for ongoing protection even as circulating antibody levels wane. Gardasil 9®’s combination of high initial antibody titters, effective CD4+ T cell responses, and durable immune memory provides broad and lasting protection against HPV-related conditions, including genital warts and cancers linked to high-risk HPV types 16 and 18. The vaccine’s ability to elicit this comprehensive immune response is central to its success in reducing HPV-related morbidity across diverse populations (36).
The Gardasil 9® vaccine elicits a robust and comprehensive immune response, targeting nine HPV types (6, 11, 16, 18, 31, 33, 45, 52, and 58) through the coordinated activation of both humoral and cellular immunity. Clinical studies demonstrated that 98.2% to 100% of individuals vaccinated with Gardasil 9 become seropositive for all nine HPV types by month 7, indicating a strong antibody response. GMTs for HPV types 6, 11, 16, and 18 are comparable to those seen with the earlier Gardasil vaccine, confirming non-inferiority in humoral immunity. This response is sustained over time, with 78% to 100% of individuals remaining seropositive for up to five years, and 81% to 98% among those vaccinated at ages 9 to 15, reflecting durable immune memory (23). The cellular immune response is equally critical, with Gardasil 9 effectively activating CD4+ T helper cells, which support antibody production and memory B cell formation, alongside CD8+ cytotoxic T cells that target HPV-infected cells (18). Key cytokines like interferon-gamma (IFN-γ) and interleukin-2 (IL-2) are upregulated, promoting a Th1-biased response that enhances cellular immunity and long-term protection (22). Moreover, the vaccine demonstrates a strong memory response, with a rapid and significant increase in antibody titers following a booster dose five years after initial vaccination, underscoring the robust immune memory established (37). This immune landscape, marked by high initial antibody levels, effective cellular responses, and key cytokine production, ensures that Gardasil 9 provides broad and lasting protection against HPV-related conditions, including genital warts and cancers associated with high-risk HPV types (24).
HPV prevalence and genotype distribution in Togo
Numerous studies have investigated the prevalence of HPV in Togo; however, these studies have employed a variety of methodological approaches over the years. These studies highlight the complexity and diversity of HPV infections in the country, particularly among vulnerable populations such as women living with HIV, female sex workers, and helminth-infected women. However, the varying methodological approaches used in HPV studies in Togo, including differences in detection methods, target populations, temporal factors and geographical focus, not only contribute to discrepancies in the overall prevalence but may also lead to variations in the distribution of HPV types, age-specific rates, and regional prevalence across different studies. In addition, the use of different sampling techniques, diagnostic criteria, and population demographics, each study may yield distinct results, highlighting the need for standardized methodologies to obtain a clearer understanding of HPV distribution in the country. Such standardization would not only enhance the reliability of the data but also facilitate more effective public health interventions aimed at reducing the burden of HPV-related diseases in Togo.
Understanding the local epidemiology of HPV is critical for assessing the potential impact of vaccines, as genotype prevalence directly influences vaccine effectiveness. In Togo, where certain high-risk HPV types may differ in prevalence from global patterns, this becomes particularly relevant. By examining the local distribution of HPV genotypes alongside vaccination efforts, we can better evaluate how well current vaccines address the strains most prevalent in this region. This alignment between genotype-specific prevalence and vaccine efficacy forms the foundation for optimizing HPV vaccination strategies in Togo.
HPV-related studies in Togo reveal a complex landscape influenced by various factors, including demographic characteristics and health status. For instance, a notable study conducted between September 2014 and September 2015 assessed HPV prevalence among HIV-infected women in Lomé, Togo. This cross-sectional study found that the overall prevalence of HPV was 22.2%. The same study identified specific high-risk HPV (HR-HPV) genotypes among the participants. The most prevalent genotypes included: HPV 18 (8.6%), HPV 68 (4.1%), and HPV 62/81 detected in 2.7%. and HPV 16 which was notably low at 1.3% (38). These findings underscore a unique distribution pattern of HPV genotypes in Togo in 2015, with HPV 18 being more prevalent than HPV 16, which is often associated with a higher risk of cervical cancer in other populations.
In 2017, another pivotal study was the first national assessment of HPV prevalence among female sex workers in Togo. This multicentric cross-sectional study involving 310 female sex workers (FSW) was conducted in four major cities of Togo: Lomé, Kara, Sokodé, and Atakpamé revealed a higher prevalence of HR-HPV at 32.9%. The most common HR-HPV types included HPV 58, HPV 35, and HPV 31. The study also noted a correlation between HIV status and HR-HPV prevalence, with higher rates observed in HIV-positive participants (39). Additionally, the findings indicated that the prevalence of HR-HPV and multiple HPV infections was significantly higher in the cohort of individuals co-infected with both HPV and HIV compared to those who were infected with HPV alone. This highlights the critical interaction of these infections and underscores the urgent need for targeted public health interventions to address the dual burden of HIV and HR-HPV among FSWs in Togo.
In 2017, another study conducted among women attending four main gynaecological clinics in Lomé, the capital city of Togo, revealed that 53.3% of the 240 participants were infected with high-risk human papillomavirus (HR-HPV). The analysis identified HPV 56 as the most prevalent genotype at 22.7%, followed closely by HPV 51 (20.3%), HPV 31 (19.5%), HPV 52 (18.8%), and HPV 35 (17.2%). In contrast, HPV 33 and HPV 16 were the least common genotypes, each detected in only 2.3% of the cases (40). These findings underscore the significant burden of HR-HPV among women in Lomé and highlight the need for targeted public health interventions, including vaccination and screening programs, to address the risks associated with HPV-related diseases.
In the same year, Dolou et al. focused on northern Togo and enrolled a total of 238 women, including those from the general population as well as women seeking care at one main gynecological clinic. For HPV screening alongside cervical lesions determination at Kara. The findings revealed an overall HR-HPV prevalence of 35.71%, with HPV 31 (18.7%), HPV 52 (13.82%), and HPV 68 (13.01%) being the most commonly detected genotypes, while HPV 16 was found to be the least frequent at 0.81% (41). These results provide valuable insights into the distribution of HR-HPV genotypes among women in Kara, Togo, which can inform the development of targeted prevention and management strategies for cervical cancer. Recently, in 2022, our team conducted a multicentric study in helminth-endemic areas of the central region of Togo revealing significant findings regarding the prevalence of helminth infections and their association with human papillomavirus (HPV) infections among women. We found that 19.2% of the participants have HPV infections, with the majority being infected with a single HPV type. The most prevalent HPV types detected in this cohort were HPV 52 (17%), HPV 45 (15%), and HPV 35 (13%). Notably, the research highlighted a substantial association between hookworm infection and an increased load of HPV infection, indicating that women with hookworm were at a higher risk of HPV infection. Surprisingly, the study found that women with co-infection of HPV and hookworm were predominantly infected with HPV type 16 (42). This finding raises significant concerns, as HPV type 16 is one of the most well-established high-risk types associated with cervical cancer. Indeed, globally, approximately 70% of cervical cancer cases are linked to infections with HPV types 16 and 18, both of which are known to play a critical role in the development of cervical carcinogenesis. In addition, the predominance of HPV 16 in women co-infected with hookworm may be attributed to immune modulation by hookworm that suppresses viral clearance, HPV 16’s high oncogenic potential and ability to evade immune detection, geographical and environmental factors increasing exposure to both infections and shared risk factors such as poor access to healthcare and lower socioeconomic status. The HPV genotype distribution in Togo is summarised in Table 2.
Table 2: HPV genotypes prevalence and distribution in Togo.
| Year | Target population | Study site | Locality (Region) | Prevalence | Most prevalent Genotypes identified | Reference | |
| 2015 | HIV infected individuals | Lomé | Maritime | 22.2% | HPV 18, 68, 62 | (43) | |
| 2017 | Female sex workers | Lomé- Tsévié Atakpamé Kara | Maritime Central Kara | 44.9%. | HPV 35, 16 | (44) | |
| 2017 | Women attending gynecological clinics | Lomé | Maritime | 53.3% | HPV 56, 51,31 | (45) | |
| 2017 | General population and gynecological clinic attendees | Kara | Kara | 35.71%, | HPV 31, 52, 68 | (46) | |
| 2022 | Hookworm infected women | Tseve Sagbadai Sakalaoudè Fazao Kikimini Alheridè | Centrale | 19.2% | HPV 52, 45, 35 | (47) | |
Implication of HPV prevalence and genotypes distribution for HPV vaccination
The choice of HPV vaccines depends on the HPV types targeted, regional prevalence, vaccine availability, cost, and public health goals, with broader protection offered by Gardasil 4® and Gardasil 9® compared to Cervarix TM, which focuses on preventing cervical cancer caused by HPV types 16 and 18. Differences in HPV prevalence can significantly affect public health strategies, particularly vaccination campaigns. Regions with higher prevalence rates of high-risk HPV types, such as HPV 16 and 18, may require more aggressive vaccination efforts to prevent cervical cancer. The presence of HPV types 16 and 18 suggests that vaccines like Cervarix could provide essential protection. However, Gardasil 9® may offer even greater benefits by covering additional high-risk types that could also contribute to cervical cancer, thereby providing a more comprehensive preventive strategy.
Variations in HPV prevalence across studies can be attributed to differences in sample sizes, populations studied, and methodologies used. For instance, studies focusing on specific demographics, such as HIV-infected women or homosexuals may report different prevalence rates compared to those examining the general population. This variability necessitates a careful analysis of local epidemiological data to guide vaccine choice effectively.
The introduction of the HPV vaccine in Togo is particularly aimed at adolescent girls aged 9 to 14, as this age group is at the ideal stage for vaccination before the onset of sexual activity. Given the high incidence of cervical cancer in women of childbearing age, a vaccine that targets the most prevalent and high-risk HPV types in this demographic is essential for maximizing the public health impact. The choice between Cervarix and Gardasil 9® should consider the local prevalence of HPV types, with an emphasis on ensuring that the selected vaccine is accessible and affordable for the target population. Gardasil 9®, with its broader coverage, may be more beneficial in regions with diverse HPV genotypes.
Effective communication strategies should be developed to inform communities about the importance of HPV vaccination and its role in preventing cervical cancer. This includes educating parents and guardians about the specific types of HPV covered by the vaccines and the significance of vaccination in protecting their daughters. Continuous monitoring of HPV prevalence and vaccination outcomes will be essential to assess the effectiveness of the chosen vaccine and to make necessary adjustments in vaccination strategies (43). This data will also help in advocating for the inclusion of the most effective vaccines in national immunization schedules. Effective vaccination strategies for human papillomavirus (HPV) infection and cervical cancer based on the mathematical model with a stochastic process have shown high success rates in preventing HPV-related diseases and cancers (44). The implications of HPV prevalence and the choice of vaccine in Togo are critical for developing an effective cervical cancer prevention strategy. By selecting a vaccine that aligns with the local epidemiological landscape and ensuring its accessibility, Togo can make significant strides in reducing the burden of cervical cancer among its population.
Concept of trained immunity and HPV vaccination
Trained immunity refers to a functional state of the innate immune system, marked by long-lasting epigenetic reprogramming of innate immune cells (45). It is a phenomenon where innate immune cells, such as macrophages and natural killer cells, exhibit enhanced responses upon re-exposure to pathogens after an initial encounter with a vaccine or infection (46). Traditionally, the lack of immunological memory has been a key factor differentiating innate immunity from adaptive immunity, but this notion is currently being involved. Indeed, unlike adaptive immunity, which relies on T and B cells and provides long-lasting, specific immunity, trained immunity involves a form of immunological memory that enhances the innate immune response to subsequent infections, potentially offering broader protection against various pathogens (47) (figure 3). Research indicates that HPV vaccines may induce trained immunity, which could provide additional benefits beyond protection against HPV itself (48). For instance, studies, have recently investigated the innate immune responses and the phenomenon of trained immunity induced by two HPV vaccines, Cervarix and Gardasil 4®, revealing that while Cervarix significantly enhances cytokine expression, Gardasil 9®, despite lower initial cytokine levels, promotes trained immunity in macrophages that enhances responses to subsequent Toll-like receptors (TLR) stimulation. The trained immunity observed was ascribed to aluminum adjuvant that robustly stimulates macrophages upon second stimulation of mice with the Gardasil 9® vaccine (49). For instance, studies have hypothesized that HPV vaccination might reduce the risk of infections such as SARS-CoV-2, this suggests that HPV vaccination could have a role in enhancing overall immune resilience in vaccinated individuals, potentially lowering the incidence of other viral infections (50).
In addition, HPV vaccines have been shown to modulate pro-inflammatory cytokine expression in response to secondary Toll-like receptor stimulations, which are crucial for initiating immune responses. This modulation indicates that the vaccine may enhance the ability of pattern recognition receptors (PRR) to respond to various pathogens, thereby contributing to the concept of trained immunity. The HPV vaccine has also been demonstrated to boost immune memory, particularly through the activation of memory B cells. These cells are essential for producing antibodies that can effectively neutralize HPV. Research has shown that even a single dose of the HPV vaccine can significantly increase the quantity and quality of HPV-specific antibodies, enhancing the immune system’s readiness to combat future infections (51). This highlights the importance of HPV vaccination not only in preventing cervical cancer and other HPV-related diseases but also in potentially offering broader immune benefits. Further studies are necessary to fully understand the mechanisms and implications of this trained immunity in the context of HPV vaccination.
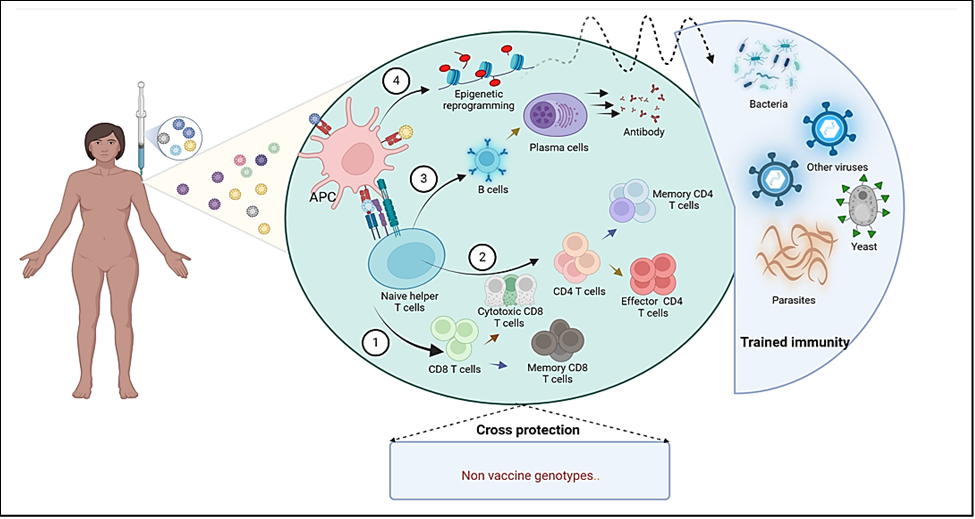
Figure 3: Immune response following HPV vaccination: The complex process involved in the immune response to HPV vaccine begins following intramuscular administration. Vaccine antigens are processed by antigen-presenting cells (APCs), which present them to naïve T cells, initiating their activation. Upon activation, naïve T cells differentiate into cytotoxic and memory CD8 T cells (1), effectors and memory CD4 T cells (2); or activated B cells that mature into Plasma cells, leading to antibody production (3). Additionally; APCs may acquire a trained memory; enhancing their ability to respond more robustly to the vaccine-specific antigens, upon subsequent stimulation, and to various unrelated pathogens, including bacteria, other viruses, and yeast (4). The available HPV vaccines may also provide cross-protection against additional genotypes not specifically targeted by the vaccine. Created with BioRender.com
Conclusion
The epidemiological assessment of HPV genotypes distribution in Togo is crucial for successfully implementing the recently initiated HPV vaccination program. By understanding the current prevalence, type distribution, and associated risk factors of HPV, we can tailor vaccination strategies to effectively target high-risk populations and regions. Cervical cancer is a preventable disease, and with the right public health strategies such as vaccination, screening, and education, its incidence can be significantly reduced. Efforts are underway globally to eliminate cervical cancer as a public health problem, particularly in regions where the burden is high. This review highlights the importance of evidence-based approaches in maximizing vaccine coverage and integration with existing cervical cancer prevention efforts. Ultimately, leveraging these in cervical cancer incidence and mortality, and improving the overall health of women in Togo.
In addition, this mini-review underscores the critical need for comprehensive local epidemiological data to refine HPV vaccination strategies in Togo. To optimize vaccination coverage and effectiveness, it is essential to focus on increasing access and targeting high-risk populations, including HIV-infected women and female sex workers. Implementing these recommendations will significantly enhance public health outcomes and promote more effective HPV prevention in the region.
Conflict of interest
Authors declare no conflicts of interest related to this work. Gnatoulma Katawa is an Editorial Board member of Women in WoSciMIB and co-author of this article. To minimize bias, he was excluded from all editorial decision-making related to the acceptance of this article for publication.
Authors contribution
G.K. conceptualized the study, supervised the project, critically reviewed and edited the manuscript. H.E.K. curated the data and drafted the original manuscript. M.K. provided critical revisions and contributed to the editing of the manuscript. All authors read and approved the final manuscript
References
- Zur Hausen H. Papillomaviruses in the causation of human cancers-a brief historical account. Virology. 2009;384(2):260-5.
- Stuebs FA, Koch MC, Dietl AK, Adler W, Geppert C, Hartmann A, et al. Cytology and high-risk human papillomavirus test for cervical cancer screening assessment. Diagnostics. 2022;12(7):1748.
- Organization WH: Human papillomavirus (HPV) and cervical cancer. 2016. June http://www who int/mediacentre/factsheets/fs380/en 2018.
- Ruddies F, Gizaw M, Teka B, Thies S, Wienke A, Kaufmann AM, et al. Cervical cancer screening in rural Ethiopia: a cross-sectional knowledge, attitude and practice study. BMC cancer. 2020;20:1-10.
- Yadav R, Zhai L, Tumban E. Virus-like particle-based L2 vaccines against HPVs: where are we today? Viruses. 2019;12(1):18.
- Mo Y, Ma J, Zhang H, Shen J, Chen J, Hong J, et al. Prophylactic and therapeutic HPV vaccines: current scenario and perspectives. Frontiers in cellular and infection microbiology. 2022;12:909223.
- Perlman S, Wamai RG, Bain PA, Welty T, Welty E, Ogembo JG. Knowledge and awareness of HPV vaccine and acceptability to vaccinate in sub-Saharan Africa: a systematic review. PloS one. 2014;9(3):e90912.
- Darré T, Djiwa T, Ladekpo KJO, M’Bortche BK, Douaguibe B, Aboubakari A-S, et al. Factors Associated With Precancerous Cervical Lesions in Human Immunodeficiency Virus–Infected Women: A Cross-Sectional Survey in Togo. Clinical Medicine Insights: Oncology. 2024;18:11795549241234620.
- Togo introduces human papillomavirus vaccine to protect adolescent girls from the leading cause of cervical cancer [https://www.gavi.org/news/media-room/togo-introduces-human-papillomavirus-vaccine-protect-adolescent-girls-leading].
- Akhatova A, Azizan A, Atageldiyeva K, Ashimkhanova A, Marat A, Iztleuov Y, et al. Prophylactic human papillomavirus vaccination: from the origin to the current state. Vaccines. 2022;10(11):1912.
- Olsson S-E, Restrepo JA, Reina JC, Pitisuttithum P, Ulied A, Varman M, et al. Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys 9 to 15 years of age: Interim analysis after 8 years of follow-up. Papillomavirus Research. 2020;10:100203.
- Shattock AJ, Johnson HC, Sim SY, Carter A, Lambach P, Hutubessy RC, et al. Contribution of vaccination to improved survival and health: modelling 50 years of the Expanded Programme on Immunization. The Lancet. 2024;403(10441):2307-16.
- Iversen O-E, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. Jama. 2016;316(22):2411-21.
- Patel C, Brotherton JM, Pillsbury A, Jayasinghe S, Donovan B, Macartney K, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Eurosurveillance. 2018;23(41):1700737.
- Rathod S, Potdar J, Gupta A, Sethi N, Dande A. Empowering women’s Health: insights Into HPV vaccination and the prevention of invasive cervical cancer. Cureus. 2023;15(11).
- Monie A, Hung C-F, Roden R, Wu TC. Cervarix™: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics: Targets and Therapy. 2008;2(1):107-13.
- Panwar K, Godi A, Cocuzza CE, Andrews N, Southern J, Turner P, et al. Binding antibody levels to vaccine (HPV6/11/16/18) and non-vaccine (HPV31/33/45/52/58) HPV antigens up to 7 years following immunization with either Cervarix® or Gardasil® vaccine. Vaccine. 2022;40(9):1198-202.
- Herrin DM, Coates EE, Costner PJ, Kemp TJ, Nason MC, Saharia KK, et al. Comparison of adaptive and innate immune responses induced by licensed vaccines for human papillomavirus. Human vaccines & immunotherapeutics. 2014;10(12):3446-54.
- Pinto LA, Dillner J, Beddows S, Unger ER. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32):4792-9.
- Zurek Munk-Madsen M, Toft L, Kube T, Richter R, Ostergaard L, Søgaard OS, et al. Cellular immunogenicity of human papillomavirus vaccines Cervarix and Gardasil in adults with HIV infection. Human vaccines & immunotherapeutics. 2018;14(4):909-16.
- Mavundza EJ, Wiyeh AB, Mahasha PW, Halle-Ekane G, Wiysonge CS. A systematic review of immunogenicity, clinical efficacy and safety of human papillomavirus vaccines in people living with the human immunodeficiency virus. Human vaccines & immunotherapeutics. 2020;16(2):426-35.
- Godi A, Panwar K, Haque M, Cocuzza CE, Andrews N, Southern J, et al. Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix® or Gardasil® vaccine. Vaccine. 2019;37(18):2455-62.
- Van Damme P, Olsson SE, Block S, Castellsague X, Gray GE, Herrera T, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136(1):e28-e39.
- Joura EA, Ulied A, Vandermeulen C, Figueroa MR, Seppä I, Aguado JJH, et al. Immunogenicity and safety of a nine-valent human papillomavirus vaccine in women 27–45 years of age compared to women 16–26 years of age: An open-label phase 3 study. Vaccine. 2021;39(20):2800-9.
- Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, et al. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer letters. 2020;471:88-102.
- Huber B, Wang JW, Roden RB, Kirnbauer R. RG1-VLP and other L2-based, broad-spectrum HPV vaccine candidates. Journal of Clinical Medicine. 2021;10(5):1044.
- HPV Vaccination Recommendations [https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html].
- Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30:F123-F38.
- Stanley M. HPV-immune response to infection and vaccination. Infectious agents and cancer. 2010;5:1-6.
- Harper DM, DeMars LR. HPV vaccines–a review of the first decade. Gynecologic oncology. 2017;146(1):196-204.
- Kjaer SK, Nygård M, Sundström K, Munk C, Berger S, Dzabic M, et al. Long-term effectiveness of the nine-valent human papillomavirus vaccine in Scandinavian women: interim analysis after 8 years of follow-up. Human vaccines & immunotherapeutics. 2021;17(4):943-9.
- Rahangdale L, Mungo C, O’Connor S, Chibwesha CJ, Brewer NT. Human papillomavirus vaccination and cervical cancer risk. Bmj. 2022;379.
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. The Lancet. 2007;369(9580):2161-70.
- Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, et al. A randomized, observer-blinded immunogenicity trial of Cervarix® and Gardasil® human papillomavirus vaccines in 12-15 year old girls. PloS one. 2013;8(5):e61825.
- Gilca V, Sauvageau C, Boulianne N, De Serres G, Crajden M, Ouakki M, et al. The effect of a booster dose of quadrivalent or bivalent HPV vaccine when administered to girls previously vaccinated with two doses of quadrivalent HPV vaccine. Human vaccines & immunotherapeutics. 2015;11(3):732-8.
- Roy V, Jung W, Linde C, Coates E, Ledgerwood J, Costner P, et al. Differences in HPV-specific antibody Fc-effector functions following Gardasil® and Cervarix® vaccination. npj Vaccines. 2023;8(1):39.
- Guevara A, Cabello R, Woelber L, Moreira Jr ED, Joura E, Reich O, et al. Antibody persistence and evidence of immune memory at 5 years following administration of the 9-valent HPV vaccine. Vaccine. 2017;35(37):5050-7.
- Nyasenu YT, Gbeasor-Komlanvi FA, Ehlan A, Issa SA-R, Dossim S, Kolou M, et al. Prevalence and distribution of Human Papillomavirus (HPV) genotypes among HIV infected women in Lomé, Togo. PloS one. 2019;14(2):e0212516.
- Ferré VM, Ekouevi DK, Gbeasor-Komlanvi FA, Collin G, Le Hingrat Q, Tchounga B, et al. Prevalence of human papillomavirus, human immunodeficiency virus and other sexually transmitted infections among female sex workers in Togo: a national cross-sectional survey. Clinical Microbiology and Infection. 2019;25(12):1560. e1-. e7.
- Kuassi-Kpede AP, Dolou E, Zohoncon TM, Traore IMA, Katawa G, Ouedraogo RA, et al. Molecular characterization of high-risk human papillomavirus (HR-HPV) in women in Lomé, Togo. BMC Infectious Diseases. 2021;21:1-7.
- Dolou ED, Kuassi-Kpede A, Zohoncon TM, Traore IM, Katawa G, Ouedraogo AR, et al. Molecular characterization of high-risk humanpapillomavirus genotypes in women with or without cervical lesions at VIA/VILI in Kara, Togo. African Health Sciences. 2021;21(4):1715-21.
- Omondi MA, Kamassa EH, Katawa G, Tchopba CN, Vogelbusch C, Parcina M, et al. Hookworm infection associates with a vaginal Type 1/Type 2 immune signature and increased HPV load. 2022;13.
- Nyasenu YT, Gbeasor-Komlanvi FA, Ehlan A, Issa SA, Dossim S, Kolou M, et al. Prevalence and distribution of Human Papillomavirus (HPV) genotypes among HIV infected women in Lomé, Togo. PLoS One. 2019;14(2):e0212516.
- Ferré VM, Ekouevi DK, Gbeasor-Komlanvi FA, Collin G, Le Hingrat Q, Tchounga B, et al. Prevalence of human papillomavirus, human immunodeficiency virus and other sexually transmitted infections among female sex workers in Togo: a national cross-sectional survey. Clin Microbiol Infect. 2019;25(12):1560.e1-.e7.
- Kuassi-Kpede AP, Dolou E, Zohoncon TM, Traore IMA, Katawa G, Ouedraogo RA, et al. Molecular characterization of high-risk human papillomavirus (HR-HPV) in women in Lomé, Togo. BMC Infect Dis. 2021;21(1):278.
- Dolou E, Kuassi-Kpede A, Zohoncon TM, Traore IM, Katawa G, Ouedraogo AR, et al. Molecular characterization of high-risk humanpapillomavirus genotypes in women with or without cervical lesions at VIA/VILI in Kara, Togo. Afr Health Sci. 2021;21(4):1715-21.
- Omondi MA, Kamassa EH, Katawa G, Tchopba CN, Vogelbusch C, Parcina M, et al. Hookworm infection associates with a vaginal Type 1/Type 2 immune signature and increased HPV load. Frontiers in Immunology. 2022;13.
- Netea MG, van der Meer JW. Trained immunity: an ancient way of remembering. Cell host & microbe. 2017;21(3):297-300.
- Yamaguchi M, Mtali YS, Sonokawa H, Takashima K, Fukushima Y, Kouwaki T, et al. HPV vaccines induce trained immunity and modulate pro‐inflammatory cytokine expression in response to secondary Toll‐like receptor stimulations. Microbiology and Immunology. 2024;68(2):65-74.
- Chen TY-T, Wang S-I, Hung Y-M, Hartman JJ, Chang R, Wei JC-C. Recent human papillomavirus vaccination is associated with a lower risk of COVID-19: a US database cohort study. Drugs. 2023;83(7):621-32.
- Pinto LA, Viscidi R, Harro CD, Kemp TJ, García-Piñeres AJ, Trivett M, et al. Cellular immune responses to HPV-18,-31, and-53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology. 2006;353(2):451-62
